The Cosmetic Fragrance And Flavor Ingredient
Right to Know Act of 2020 (SB 312)
Compliance Information for Companies
Senate Bill 312 (SB 312), now known as the Cosmetic Fragrance and Flavor Ingredient Right to Know Act of 2020 (CFFIRKA), goes into effect on January 1, 2022. CFFIRKA can be found in
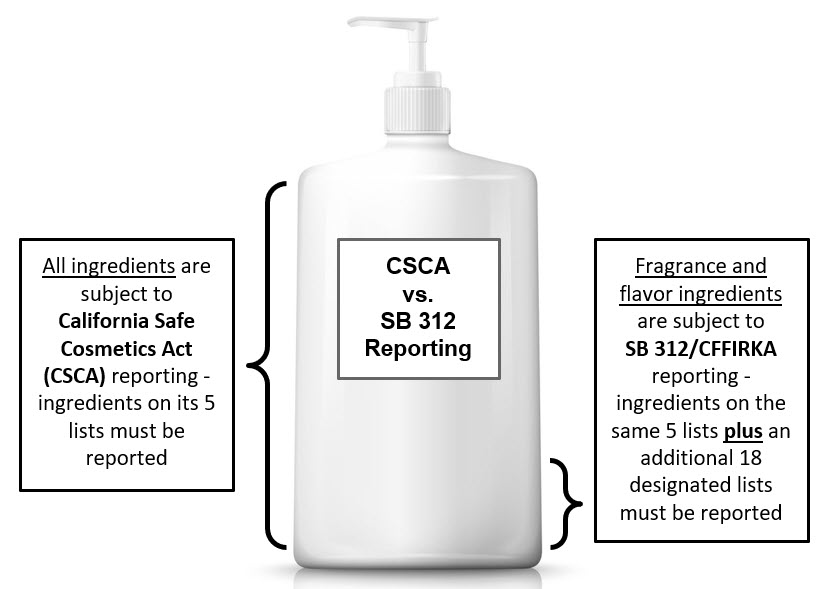
Health and Safety Code Section 111792.6. As of that date, cosmetic products sold in the state must be reported to the California Safe Cosmetics Program (CSCP) if they contain reportable fragrance and flavor ingredients. When one of the 23 designated lists that comprise the reportable ingredients lists is updated, companies have 6 months to comply from the date of adoption or effective date, whichever is later. CSCP has updated the
CSCP Reportable Ingredients List to include the lists designated in CFFIRKA.
The subset of CFFIRKA reportable ingredients called "fragrance allergens" have a distinct reporting requirement. Fragrance allergens, unlike other fragrance ingredients, must be reported regardless of their intended purpose in the product, i.e. they must be reported even if they are not used to impart scent or counteract odor. Additionally, fragrance allergens only need to be reported if they are present in a rinse-off cosmetic product at a concentration at or above 0.01 percent (100 parts per million) or in a leave-on cosmetic product at a concentration at or above 0.001 percent (10 parts per million). Fragrance allergen ingredients are clearly distinguished in the CSCP Reportable Ingredients List. All other ingredients appearing on the Reportable Ingredients List must be reported regardless of concentration in the product.
The
California Safe Cosmetics Reporting Portal previously used only for California Safe Cosmetics Act (CSCA) reporting was updated to accommodate CFFIRKA reporting as well. If you cannot find the information you are seeking here, please check the
CSCA & Reporting Portal FAQ, the Cosmetic Companies page, or email SafeCosmetics@cdph.ca.gov.