The purpose of this letter is to notify you of a potential threat to patient safety if your facility uses parenteral medications for multiple patients with unsafe injection practices. In early 2008, an outbreak of hepatitis C infection was detected in six persons who received anesthesia at an endoscopy center in Nevada. An investigation indicated that the infected patients were most likely exposed to hepatitis C in the following manner:
A clean syringe and needle were used to draw sedative from a new vial of a sedative; this was a single-use medication vial.
The sedative was then administered to a hepatitis C infected patient and backflow of blood from the patient into the syringe presumably contaminated the syringe with hepatitis C virus, not necessarily visible to the healthcare worker.
The needle was replaced on the syringe with a new, sterile needle, but the syringe was reused to draw additional sedative from the same vial for the same patient, presumably contaminating the vial with blood containing hepatitis C virus.
A clean needle and syringe were used for subsequent patients, but the contaminated vial was reused, exposing subsequent patients to hepatitis C virus.
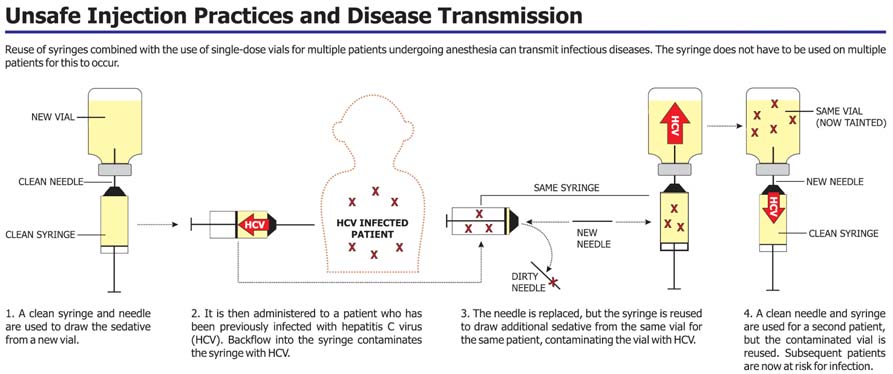
Source: Southern Nevada Health District
Although these practices have since been corrected, they were the prevailing practices of the clinic for an extended period and nearly 40,000 Nevada residents have been notified by letter to recommend that they visit their primary care provider to be tested for hepatitis C, hepatitis B, and HIV.
Numerous outbreaks of hepatitis B and hepatitis C among patients in ambulatory care facilities in the United States have identified a need to reinforce safe injection practices. In addition to endoscopy clinics, these have occurred in private medical practices, pain clinics, and a hematology/oncology clinic. Outbreaks related to unsafe injection practices indicate that some healthcare personnel are unaware of, do not understand, or do not adhere to basic principles of infection control and aseptic technique. A survey of U.S. healthcare workers who provide medication through injection found that 1% to 3% reused the same needle and/or syringe on multiple patients. Among the deficiencies identified in these outbreaks were a lack of oversight of personnel and failure to follow-up on reported breaches in infection control practices in ambulatory settings. Therefore, to ensure that all healthcare workers understand and adhere to recommended practices, principles of infection control and aseptic technique need to be reinforced in training programs and incorporated into institutional polices that are monitored for adherence.
We strongly recommend that all healthcare facilities immediately determine if multidose medication vials, or multiple use of single–use vials, are used in your facility and that injection practices be observed. One hospital recently discovered that multidose lidocaine vials were used for placement of intravenous lines prior to surgery. These vials were not purchased through the pharmacy and were being used without the knowledge of infection control personnel. Therefore we recommend visual inspection of any unit where multidose vials are likely to be used, particularly where local anesthetics, insulin, heparin, or vaccines are used. Use of single-dose medication vials should be observed to ensure they are not used for multiple patients. Personnel administering these medications should be observed for current injection practices and asked about past practices. Injection practices should be consistent with CDC recommended safe injection practices that are part of Standard Precautions (see attached). A CDC fact sheet regarding syringe reuse is also attached.
Whenever possible, the Centers for Disease Control and Prevention (CDC) and the California Department of Public Health (CDPH) recommend that single-use vials be used, that single-use vials never be used for multiple patients, and that multidose vials of medication be assigned to a single patient to reduce the risk of disease transmission.
If you have any questions about the use of multidose medication vials or safe injection practices please contact Jon Rosenberg, M.D. (jon.rosenberg@cdph.ca.gov) or Sue Chen (sue.chen@cdph.ca.gov) at (510) 620-3434.
Sincerely,
Original Signed by Kathleen Billingsley, R.N.
Kathleen Billingsley, R.N.
Deputy Director
Attachments
Safe Injection Practices
The following recommendations apply to the use of needles, cannulas that replace needles, and, where applicable intravenous delivery systems
- Use aseptic technique to avoid contamination of sterile injection equipment.
- Do not administer medications from a syringe to multiple patients, even if the needle or cannula on the syringe is changed. Needles, cannulae and syringes are sterile, single-use items; they should not be reused for another patient nor to access a medication or solution that might be used for a subsequent patient.
- Use fluid infusion and administration sets (i.e., intravenous bags, tubing and connectors) for one patient only and dispose appropriately after use. Consider a syringe or needle/cannula contaminated once it has been used to enter or connect to a patient’s intravenous infusion bag or administration set.
- Use single-dose vials for parenteral medications whenever possible.
- Do not administer medications from single-dose vials or ampules to multiple patients or combine leftover contents for later use.
- If multidose vials must be used, both the needle or cannula and syringe used to access the multidose vial must be sterile.
- Do not keep multidose vials in the immediate patient treatment area and store in accordance with the manufacturer’s recommendations; discard if sterility is compromised or questionable.
- Do not use bags or bottles of intravenous solution as a common source of supply for multiple patients.
From Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings 2007, Standard Precautions (
http://www.cdc.gov/ncidod/dhqp/gl_isolation_standard.html).
Fact Sheet
A Patient Safety Threat – Syringe Reuse
Patients need to be aware of a very serious threat to their health – the reuse of needles or syringes, and the misuse of medication vials. Healthcare providers (doctors, nurses, and anyone providing injections) should never reuse a needle or syringe either from one patient to another or to withdraw medicine from a vial. Both needle and syringe must be discarded once they have been used. It is not safe to change the needle and reuse the syringe – this practice can transmit disease.
Figure 1. Picture of a needle and syringe.
A single-use vial is a bottle of liquid medication that is given to a patient through a needle and syringe. Single-use vials contains only one dose of medication and should only be used once for one patient, using a clean needle and clean syringe.
 A multidose vial is a bottle of liquid medication that contains more than one dose of medication and is often used by diabetic patients or for vaccinations. A new, clean needle and clean syringe should always be used to access the medication in a multidose vial. Reuse of needles or syringes to access medication can result in contamination of the medicine with germs that can be spread to others when the medicine is used again.
A multidose vial is a bottle of liquid medication that contains more than one dose of medication and is often used by diabetic patients or for vaccinations. A new, clean needle and clean syringe should always be used to access the medication in a multidose vial. Reuse of needles or syringes to access medication can result in contamination of the medicine with germs that can be spread to others when the medicine is used again.
Figure 2. Picture of a multidose vial.
Whenever possible, CDC recommends that single-use vials be used and that multidose vials of medication be assigned to a single patient to reduce the risk of disease transmission.
Healthcare providers should always adhere to Safe Injection Practices under Standard Precautions to prevent disease transmission from needles, syringes, or multidose vials.
Reusing a needle or syringe puts patients in danger of contracting hepatitis B, hepatitis C, and possibly HIV. When it is discovered that reuse of a needle or syringe has occurred, all patients who may have been affected should be notified and informed to get tested.
From: Centers for Disease Control and Prevention (CDC) Division of Healthcare Quality Promotion (https://www.cdc.gov/injectionsafety/patients/syringereuse_faqs.html#).